Curved π-System with Intense Solid-State Emission Behavior
|
Published in ”Angewandte Chemie International Edition” (Online Publication, June 18, 2015). |
Prof. Murata, Y., Assoc Prof. Wakamiya, A., Assist Prof. Murata, M. (From Left)
Mr. Chaolumen, Mr. Sugano, Y (From Left) |
|||
|
Assist Prof. Murata, M., Mr. Chaolumen, Mr. Sugano, Y., (Division of Synthetic Chemistry, Structural Organic Chemistry) |
||||
|
Assist Prof. Michihisa Murata, Mr. Chaolumen, Mr. Yasunori Sugano, Assoc Prof. Atsushi Wakamiya, and Prof. Yasujiro Murata in Institute for Chemical Research have elucidated unique reactivity of a PAH containing two unsaturated pentagon rings and created a curved π-system with intense emission behavior in the solid state. |
||||
|
Polycyclic aromatic hydrocarbons (PAHs) containing fully unsaturated five-membered rings, so called cyclopenta-fused polycyclic aromatic hydrocarbons (CP-PAHs), have received attention because of their unusual properties such as high electron affinities and unique chemical reactivities, as well as for their potential use in organic electronic devices. However, knowledge on the CP-PAHs has still been limited due to the difficulty in preparation of a variety of derivatives based on CP-PAHs in large quantities. |
||||
|
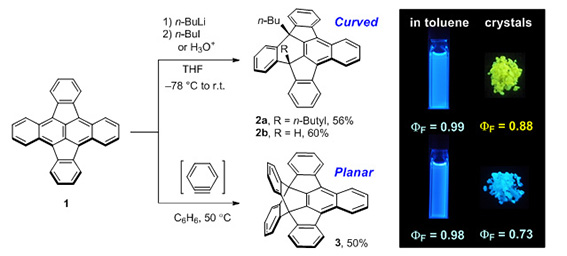
Figure 1. Conversions of CP-PAH 1 into curved and planar π-systems 2 and 3 with distinct emission behaviors. |
||||
|
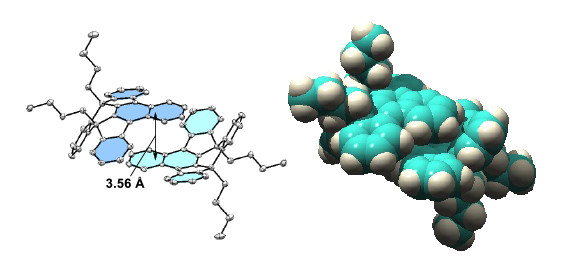
Although compounds 2a and 3 exhibited similar sky-blue emissions in a dilute solution, the emission band of 2a was significantly red-shifted in the solid state and exhibited intense yellow emission, while 3 retained the intense blue color emission in the solid state. The XRD analysis demonstrated that 2a forms a dimeric structure between the concave surfaces with effective π-overlap (Figure 2), which can cause a significant red-shift in the fluorescence by the stabilization of excimer. These findings can provide ways toward solid-state fluorescent materials with intriguing 3D structures. |
||||
|
Figure 2. The face-to-face dimeric structure between the concave surfaces of 2a in the crystal. |
||||
|
This work was supported by Grant-in-Aid for Scientific Research (B) (Grant Number 24350024) from JSPS. The synchrotron radiation experiments were performed at the BL38B1 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2012B1319 and 2013A1489). This work was carried out with the NMR spectrometer in the Joint Usage/Research Center (JURC) at ICR, Kyoto University. |
||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center