Investigation of Organoiron Catalysis in Kumada–Tamao–Corriu-Type Cross-Coupling Reaction Assisted by Solution-Phase X-ray Absorption Spectroscopy
|
Published in “Bulletin of the Chemical Society of Japan“(Online Publication, March 15, 2015).
|
Prof. Nakamura, M., Assist Prof. Isozaki, K., Program-Specific Assist Prof. Iwamoto, T., Mr. Nakajima, S., Mr. Yoshida, R., Assoc Prof. Takaya, H., Dr. Adak, L. (clockwise from the one on the far left) |
|||
|
Assoc Prof. Takaya, H., Mr. Nakajima, S., Mr. Nakagawa, N., Assist Prof. Isozaki, K., Program-Specific Assist Prof. Iwamoto, T., Mr. Imayoshi, R., Dr. Gower, N. J., Dr. Laksmikanta, A., Assist Prof. Hatakeyama, T.1, Prof. Nakamura, M. (International Research Center for Elements Science, Organic Main Group Chemistry) (1:presently Associate Professor in Kwansei University and ESICB)
Dr. Honma, T., Dr. Masafumi, T. (Japan Synchrotron Radiation Research Institute (JASRI, SPring-8))
Assist Prof. Sunada, Y., Prof. Nagashima, H. (Division of Applied Molecular Chemistry, Institute for Materials Chemistry and Engineering, Kyushu University)
Dr. Hashizume, D. (Center for Emergent Matter Science, RIKEN)
Dr. Takahashi, S. (Institute for Sustainable Sciences and Development, Hiroshima University) |
||||
|
Professor Masaharu Nakamura, Associate Professor Hikaru Takaya and their research group has succeeded to elucidate a precise mechanism of iron-catalyzed cross-coupling reaction, which is a long-standing problem in iron catalysis from the Kochi’s first discovery of iron-catalyzed cross-coupling reaction in 1971. |
||||
|
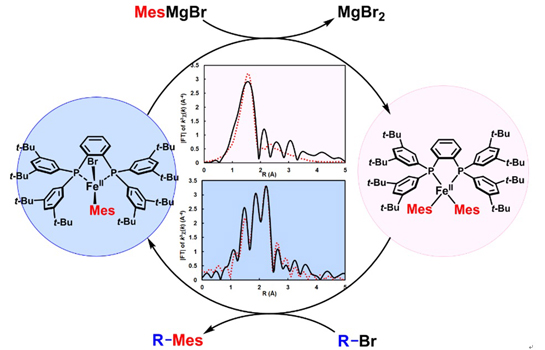
Their study aimed to identify the catalytically active organoiron intermediates formed in the iron bisphosphine complex-catalyzed Kumada–Tamao–Corriu type cross-coupling reaction (KTC cross-coupling), which are almost invisible to the conventional NMR and EPR spectroscopies. The effectiveness of solution-phase XAFS analysis for investigating these organoiron species were successfully demonstrated. Under the KTC cross-coupling condition with Fe(+2)X2(SciOPP) and mesitylmagnesium borimide, oxidation states and coordination geometries of the resulting Fe(+2)BrMes(SciOPP) and Fe(+2)Mes2(SciOPP) complexes were able to determined using solution-phase X-ray absorption near-edge structure (XANES) spectroscopy, and the solution-phase molecular structure of these organoiron species were also confirmed using extended X-ray absorption fine structure (EXAFS) analysis. |
||||
|
Figure 1. The reaction mechanism for FeX2SciOPP catalyst determined by XAFS study. |
||||
|
Nowadays, iron-catalyzed cross-coupling reactions have greatly impacted organic synthesis because of non-classical reactivities, such as for sp2–sp3 and sp3–sp3 cross-coupling reactions, in comparison with the conventional Pd-catalyzed cross-coupling reactions. Despite the practical advantages of iron, for example, low cost, low toxicity, high abundance on the earth, and easy separation of metal residue due to the lower ionization energy, the catalytic use of iron has been overlooked for 30 years since Kochi’s first discovery of iron-catalyzed cross-coupling reaction. The principal factor responsible for this backward is the strong paramagnetic property of iron which interferes with conventional solution-phase NMR- and ESR-based mechanistic studies.
On the other hand, the Novel Prize chemistry of Pd-catalyzed cross-coupling reaction, and also the Ru-catalyzed methasesis, hydrogenation, dramatic progress had been achieved along with the prevailing of high-field FT-NMR equipped with superconducting magnet in the 1980s through 1990s, because the commonly diamagnetic low-spin 4d-transition-metal complexes well facilitate for solution-phase NMR-based characterization in the organic reaction mixture. |
||||
|
For XAFS-based study of catalysis, it has been well-established and a mature technique for mechanistic study of heterogeneous catalysts. In 1981, Stults, Friedman, Knowles, Lytle, and co-workers demonstrated the effectiveness of solution-phase XAS technique for the investigation of catalytic intermediates in the Novel Prized-Rh-catalyzed hydrogenation; however, the extremely limited accessibility to the strong X-ray source required for solution-phase SAX and the infant methods of XAS spectrum analysis had hampered the widespread use of XAS among chemists. In this decade, the situation has changed.
The easy access to 3rd generation synchrotron facilities provides more opportunities for convenient use of XAS technology by all chemists. The high brilliant and coherent X-ray provides enables us to carry out high resolution and excellent SN ratio solution-phase XAS analysis for the investigation of homogeneous catalysts in organic media.
So recently there have been an excellent pioneering studies of XAS-based investigation of homogeneous iron salts-catalyzed reactions by Bauer and co-workers. However the nature of iron complex, easy formation of clusters, a large equilibrium constant of ligand exchange along with disproportionation, and the instability of organoiron species toward oxygen, moisture, and heat, complicates XAFS spectrum and the XAFS-based solution-phase structure elucidation of organoiron species has been still a challenging issue in the area of oganometallic catalysis. |
||||
| The key to success of this research is the combination of solution-phase XAS and rapid single crystal X-ray analysis which affords the atomic coordinates of unstable organoiron complexes. Accurate EXAFS fitting simulation and DFT-level core excitation calculation for XANES pre-edge assignment made it easy to analyze XAFS spectrum of high spin state organoiron. We also confirmed that the DFT-based atomic coordinates, obtained by B3LYP* with 6-31G** calculation under considering spin state and electron configuration, can be used for accurate XAS analysis.
This result suggests that structure elucidation of crystallographically undefined organoiron species in homogeneous reaction mixture can be carried out by the combination of solution-phase XAS and DFT calculation. We consider that the wide scope of observable nuclei in XAS spectroscopy provides most practical method to investigate other paramagnetic organometallic catalyst such as Mn, Co, Ni, and Cu complexes all of which are significantly important metal for element strategy. |
||||
|
This work was supported by “Funding Program for Next generation World-Leading Researchers (Next Program)” initiated by the Council for Science and Technology Policy (CSTP), CREST (Nos. 1102545 and 11103784) programs from Japan Science and Technology Agency (JST), a Grant-in-Aid for Scientific Research (No. 22550099) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) through the JSPS, and the JURC at ICR, Kyoto University. S.N. expresses his deep gratitude to the Sumitomo Fellowship program. N.N. expresses his special thanks to the ICR exchange program going abroad.
|
||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center