Thiazole-Fused Benzothiadiazole as a Key Skeleton for T-Shaped Electron-Accepting Building Blocks
|
Assoc Prof. Wakamiya, A., Mr. Satou, M., Prof. Murata, Y. (Structural Organic Chemistry, Division of Synthetic Chemistry) |
Prof. Murata, Y., Assoc Prof. Wakamiya, A., Mr. Satou, M(from Left) |
||||
| Assoc Prof. Atsushi Wakamiya, Mr. Motoi Satou (graduate student), and Prof. Yasujiro Murata at the Institute for Chemical Research, Kyoto University, have developed T-shaped electron-accepting units as a key skeleton for organic photovoltaic materials with high solubility in common organic solvent. | |||||
|
Organic photovoltaics (OPVs) have received much attention as a next generation solar cells because of their potential of cost-effective fabrication by printing process. In order to improve the photon-to-electricity conversion efficiency of OPVs, the development of sophisticated organic semiconducting materials with both high carrier mobility and intense and broad light absorption characteristics are strongly required. In the molecular design of such organic materials, construction of rigid and planar skeleton is a promising strategy to realize high carrier mobility, which would be induced by dense packing structure in solid state. However, such rigid and planar molecules generally show low solubility in the common solvent, thus being difficult to be applied to printing process. |
|||||
|
Figure 1. Molecular design concept of soluble T-shaped electron-accepting unit (SaT). |
|||||
|
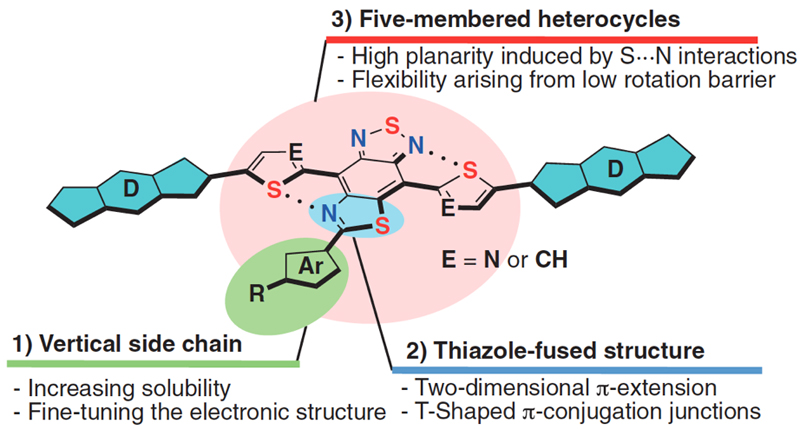
As a skeleton having both high planarity in the solid state and high solubility in organic solvents, the research group designed a T-shaped electron-accepting unit composed of thiazole-fused benzothiadiazole (Figure 1).
In summary, novel T-shaped electron-accepting units based on thiazole-fused benzothiadiazole skeletons (SaT) were designed and synthesized. These compounds are highly soluble in solution and exhibit high planarity in the solid state as evident from the structural analysis. The electrochemical and photophysical properties of these benzothiadiazole units demonstrated that thiazole-fusion induces high levels of electron-accepting ability, which can be easily tuned. These thiazole-fused benzothiadiazoles moreover exhibit dual absorption properties based on an effective two-dimensional π-expansion. Further development of D-A materials based on these T-shaped benzothiadiazole building blocks and their device applications in organic electronics are currently in progress in their laboratories. |
|||||
|
Acknowledgement This research was partially supported by the Center of Innovation Program and the PRESTO project from Japan Science and Technology Agency, JST, and JSPS KAKENHI Grant Number 26288093. |
|||||
|
These results were introduced in “The Business & Technology Daily News” (26, March, 2014, page 26) and will be published in “Chemistry Letters“, 2014. |
|||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center