Successful Synthesis of the Most Strained “Carbon Nano Ring”
|
Published in “Journal of the American Chemical Society“(Online Publication, January 27, 2014).
Program-Specific Assist Prof. Kayahara, E., Dr. Patel, V. K.,
Prof. Yamago, S., Program-Specific Assist Prof. Kayahara, E. and Dr. Patel, V. K. (Polymer Controlled Synthesis, Division of Materials Chemistry)
|
|
Professor Shigeru Yamago, Program-Specific Assistant Professor Eiichi Kayahara and Dr. Vijay Patel Kumar in Institute for Chemical Research have employed synthesis of the most strained “Carbon Nano Ring”. |
|
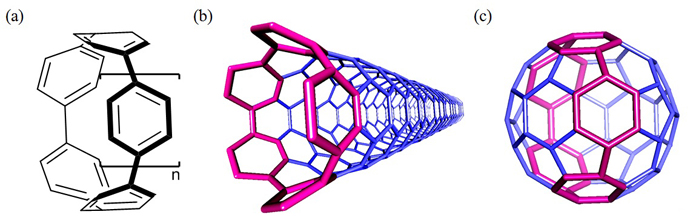
Cycloparaphenylenes (CPPs) (Figure 1a) which consist of phenylene units that are para-linked in a cyclic manner, have attracted increasing attention for not only their aesthetic structure having radially extended unique p-orbitals originating from the curvature but also their applications in electronic and optoelectronic materials, as CPPs are the smallest structural units of the sidewalls of armchair carbon nanotubes (CNTs) (Figure 1b) and structural constituents of fullerenes (Figure 1c). Despite their simple structure, however, the synthesis of CPPs had been a significant challenge due to the difficulty in constructing the highly strained cyclic structure. After desperate endeavor over more than a half century, the synthesis of CPPs was achieved recently by three groups including our own and substantial progress has been described in chemical synthesis for hoop-shaped -conjugated molecules related to CPPs. Theoretical studies by our group suggested that, CPPs of small ring size should display a range of intriguing electronic properties compared to larger CPPs. In particular, the calculated HOMO-LUMO energy gap of [5]CPP, which is a constituent of the equatorial belt of C60 (Figure 1c), is almost identical to that of C60, suggesting that [5]CPP would be an excellent lead compound for molecular electronics. However, synthesis of [5]CPP has not been reported so far. |
|
Figure 1. Structures of (a) cycloparaphenylenes (n = 1 for [5]CPP), (b) (5,5) armchair CNT, and (c) C60. The [5]CPP units in the CNT and C60. |
|
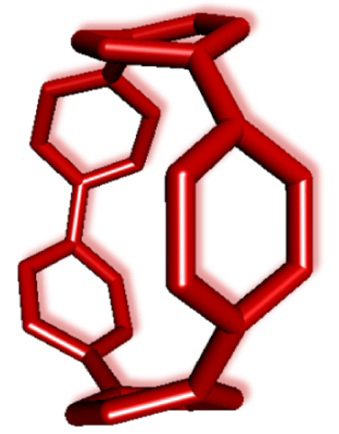
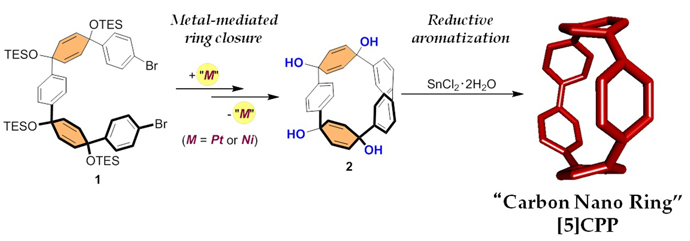
Our research group reported the synthesis of CPPs based on the platinum-mediated assembly of 4,4’-biphenyl unit forming a square-shaped cyclic platinum complex, followed by the reductive elimination of the platinum. In this paper, we succeeded to synthesize [5]CPP for the first time via new synthetic route, which was combination of our own and US group’s synthetic methods. Starting from triethylsilyl (TES)-protected masked precursor 1, cyclized product 2 was obtained through metal-mediated ring closure by nickel or platinum complexes. Then, the deprotection of TES group and subsequent reductive aromatization successfully afforded [5]CPP as a dark purple solid. [5]CPP was stable under air and was soluble in many common solvents. Moreover, the optical and electrochemical studies revealed that narrow HOMO-LUMO gap of [5]CPP is comparable to that of C60 as predicted by the theoretical calculation. The results suggest that [5]CPP should be an excellent lead compound for molecular electronics. |
|
Figure 2. Synthesis of the most strained “carbon nano ring”, [5]CPP |
|
In summary, [5]CPP was synthesized for the first time by the metal-mediated ring closure of TES-protected masked precursor followed by the deprotection of TES group and subsequent reductive aromatization. The synthetic method would be applicable to the synthesis of larger CPPs under mild conditions, and such a synthetic route should be suitable for large-scale synthesis. The optical and electrochemical studies suggest that [5]CPP should be an excellent lead compound for charge-transporting material in organic electronics such as organic light-emitting diodes, organic semiconductors, and organic solar cells. Furthermore, since [5]CPP is expected to have high chemical reactivity, chemical modification of [5]CPP for the synthesis of functionalized CPPs would be of great interest. |
|
This work was partly supported by the CREST program of the Japan Science and Technology Agency (S.Y.) and by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (E.K.). |
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center