Prof. Yamago, S., Program-Specific Assist Prof. Kayahara, E. and Mr. Iwamoto, T., and Assoc Prof. Takaya, H. “Successful Synthesis of a Ball-like Three-Dimensional π-Conjugated Molecule” (Published in “Nature Communications” 29 October 2013)
|
Successful Synthesis of a Ball-like Three-Dimensional π-Conjugated Molecule
Published in “Nature Communications“(Online Publication, October 29, 2013).
Prof. Yamago, S., Program-Specific Assist Prof. Kayahara, E. and Mr. Iwamoto, T. (Polymer Controlled Synthesis, Division of Materials Chemistry)
Assoc Prof. Takaya, H. (Organic Main Group Chemistry, International Research Center for Elements Science) |
Program-Specific Assist Prof. Kayahara, E., Mr. Iwamoto, T.,Assoc Prof. Takaya, H.,Prof. Yamago, S. (from Left) |
|||
| Professor Shigeru Yamago, Program-Specific Assistant Professor Eiichi Kayahara, Mr. Takahiro Iwamoto, and Associate Professor Hikaru Takaya in Institute for Chemical Research have employed Synthesis and physical properties of a ball-like three-dimensional π-conjugated Molecule. | ||||
|
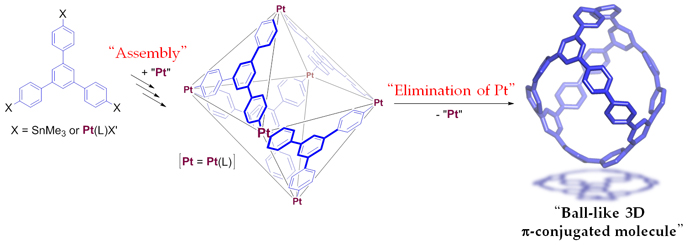
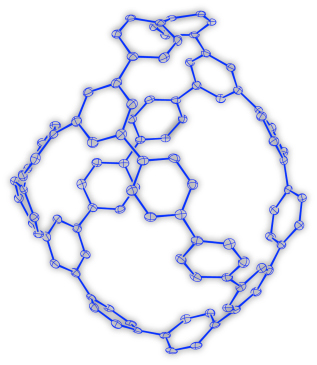
Curved π-conjugated molecules with closed and three dimensional (3D) structures, such as fullerenes and carbon nanotube have been the subject of intensive research due to their potential applications in molecular electronics. However, basic molecular skeletons of 3D molecules are limited because of the lack of a rational and selective synthetic method by organic synthesis. Our research group succeeded in the synthesis of 3D π-conjugated molecule based on a new synthetic strategy, which involves the platinum-mediated assembly of π-units by transmetallation and subsequent reductive elimination of the platinum (Figure 1). The assembly process, by which the hexanuclear platinum complex was formed, mimics the self assembly of metal and ligands that form structurally related cage-like coordination complexes. Furthermore, despite the high strain of a ball-like molecule, reductive elimination of platinum from the hexanuclear platinum complex proceeded with reasonable yield. The structure of the molecule was unambiguously determined by the single-crystal X-ray analysis using the single crystal analysis beam lines (BL02B1) at SPring-8 (Figure 2). In addition, the optical and electrochemical properties and charge mobility of the molecule are reported. As many metal–organic frameworks with 3D structures are already known and can easily be prepared, the assembly/reductive elimination strategy described in this paper would provide a variety of new 3D π-conjugated molecules with different structures and topologies, which are challenging to obtain using conventional synthetic methods. In view of the diverse range of possible functions of π-conjugated molecules, the current method has the potential to open up a new era of 3D π-conjugated molecules. |
||||
|
Figure 1. Synthesis of a Ball-like 3D π-Conjugated Molecule. |
||||
|
Figure 2. Crystal structure of a Ball-like 3D π-Conjugated Molecule. Thermal ellipsoids are shown at 50% probability. Hydrogen atoms and solvent atoms are omitted for clarity. |
||||
| This work was partly supported by the CREST program of the Japan Science and Technology Agency (S.Y.) and by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (E.K.). Single-crystal X-ray analysis was performed at BL02B1 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (2012B1790). | ||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center