Assist Prof. Agou, T., Mr. Wasano, T., and Prof Tokitoh, N., et. al. “Syntheses and Structures of an”Alumole” and Its Dianion” (Published in “Angewandte Chemie International Edition” 8 August 2013)
|
Syntheses and Structures of an”Alumole” and Its Dianion
Published in “Angewandte Chemie International Edition“(Online Publication, August 8, 2013).
Assist Prof. Agou, T., Mr. Wasano, T., and Prof Tokitoh, N. (Organoelement Chemistry, Division of Synthetic Chemistry) Dr. Jin, P., and Prof Nagase, S. (Fukui Institute for Fundamental Chemistry, Kyoto University) |
Assist Prof. Agou, T., Mr. Wasano, T., and Prof Tokitoh, N. (from Left) |
|||
|
The first Lewis base-free alumole and its dianion have been synthesized. Their structures and properties have been elucidated experimentally and theoretically. |
||||
|
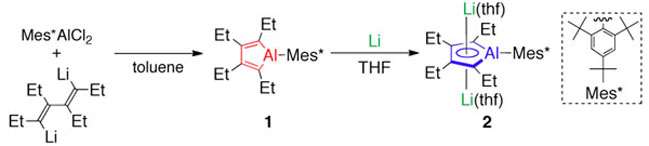
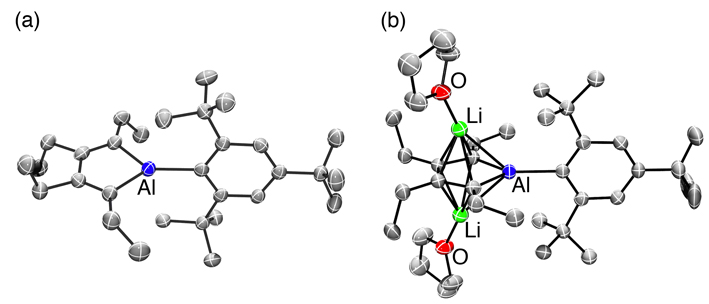
Heteroles of group 13 elements are expected to possess low-lying LUMOs because of the p– π* conjugations. Various borole derivatives have been synthesized, while only a few heavier group 13 heteroles have been reported. Furthermore, their aluminum analogues, i.e, alumoles (aluminacyclopentadienes), have yet to be synthesized so far. Herein, the first Lewis base-free alumole 1 has been synthesized by using a bulky aryl substituent, the Mes* group. X-Ray crystallographic analysis of 1 revealed that the butadiene moiety in the AlC4 ring exhibits apparent bond alternation. Treatment of the alumole with lithium afforded the lithium salt of the alumole dianion 2. In the crystal structure of lithium salt 2, the AlC4 ring is sandwiched by two lithium atoms, resulting in an inverse sandwich structure, and the C–C bond lengths in the AlC4 ring are almost equalized. DFT calculations revealed that the coordination of the lithium cations to the alumole dianion enforces the planar geometry of the AlC4 ring. |
||||
|
Figure 1. Syntheses of alumole 1 and its dianion 2. |
||||
|
Figure 2. Crystal structures of (a) alumole 1 and (b) dianion 2 (50% probability level). |
||||
|
This work was partially supported by JSPS KAKENHI Grant (Nos. 22350017, 24550048, 24655028, and 24109013) and by the “Molecular Systems Research” project of RIKEN Advanced Science Institute. We acknowledge the financial support by the Kyoto Technoscience Center. |
||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center