Prof. Yamago, Mr. Iwamoto, and coworkers, ”Size-selective Encapsulation of C60 by Cycloparaphenylene” (Published in Scientific Reports, 18 July 2011)
|
Prof. Yamago, Mr. Iwamoto, and coworkers (Polymer Controlled Synthesis, Division of Materials Chemistry)
Size-selective Encapsulation of C60 by Cycloparaphenylene
Published in Angewandte Chemie International Edition(Online Publication, July 18, 2011). |
Prof. S. YAMAGO and Mr. T. Iwamoto |
| Dr. S. Yamago (Prof.), Mr. T. Iwamoto (Doctor course student), Mr. Y. Watanabe(Alumni, presently, NIPPON SHOKUBAI CO., LTD.), Mr. T. Sadahiro (Alumni, in Hiroshima University), and Dr. T. Haino (Prof. in Hiroshima University) discovered highly size-selective complexation of C60 by cycloparaphenylenes. This type of complementary host-guest chemistry will be useful for the size- and shape-selective separation of higher fullerenes and carbon nanotubes. | |
|
Cycloparaphenylenes (CPPs) are cyclic π-conjugated molecules and possess smallest structural unit of armchair carbon nanotube (CNT). Despite the extensive synthetic challenge for more than a half-century, CPPs became available only recently. It is now anticipated the rapid progress of new researches of CPPs including the elucidation of basic properties and the application to materials science. |
|
| From the analogy of the existence of layered carbon networks with a curved surfaces, for example multi-walled carbon nanotubes and fullerene-peapods, the concave cavity of the CPPs should act as a host for π-conjugated molecules with a convex surface, such as fullerenes. Such a host-guest complex would be a suitable model for elucidating convex-concave π-π interactions. Based on our synthetic studies of CPPs, we now found for the first time that [10]CPP, which possesses 10 benzene unit, selectively encapsulate C60 forming [10]CPP⊃C60 (Scheme 1). |
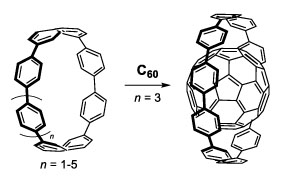
Scheme1. Size selective encapsulation of C60 by [10]CPP. |
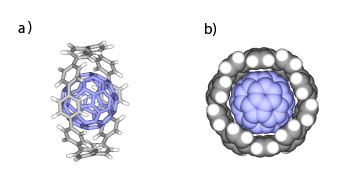
Scheme2. Optimized structure of a) [10]CPP⊃C60 complex (side view) and b) its CPK model (top view). |
The interaction between [10]CPP and C60 is very strong, and [10]CPP is one of the strongest host for C60 consisting of simple hydrocarbons. Theoretical calculation suggests that [10]CPP possesses appropriate size for C60 to maximize concave-convex π-π interactions (Scheme 2). This type of complementary host-guest chemistry will be useful for the size- and shape-selective separation of higher fullerenes and CNTs. |
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center