Professor NAKAMURA, Masaharu and his research group “Development of Iron-Catalyzed Suzuki-Miyaura Coupling Reaction of Alkyl Halides” (Published in “Journal of the American Chemical Society,” 20 July 2010)
|
Professor NAKAMURA, Masaharu and his research group (Laboratory of Organic Main Group Chemistry, International
“Development of Iron-Catalyzed Suzuki-Miyaura Coupling Reaction of Alkyl Halides“
Published in “Journal of the American Chemical Society,” |
|
|
Takuji Hatakeyama, Toru Hashimoto, Hirofumi Seike, Hikaru Takaya, Teruo Ono, Masaharu Nakamura |
|
|
Suzuki–Miyaura coupling reaction is one of the most important carbon–carbon bond forming reactions and the founder of the reaction, Professor Akira Suzuki, was awarded the Novel Prize in 2010. Development of the named reaction with the aid of palladium catalysis has facilitated synthesis of drugs, agrochemicals, liquid crystals, electronic materials in academia as well as industry. Despite these application profile and maturity, possible alternatives based on cheaper and/or less toxic catalysts has been desired and thus intensive efforts have been paid to develop next-generation catalyst system in recent years. |
|
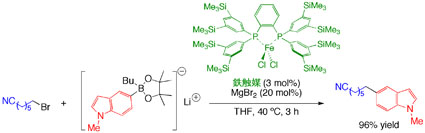
| We envisaged that the development of novel Suzuki–Miyaura coupling reaction based on iron catalysis, would be an ideal process for material synthesis from the viewpoint of economy and sustainability. Although our initial attempts using conventional iron catalysts and typical base additives did not promote the reaction at all, we finally found that lithium arylborate prepared from arylboronic acid pinacol ester and alkyllithium can be effectively cross-coupled with alkyl halides in the presence of a catalytic amount of a novel iron-phsphine complex which we have designed and synthesized recently. | 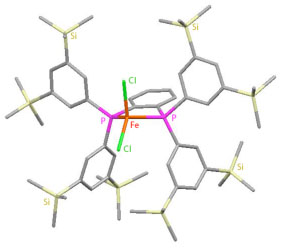 |
|
Since the iron-catalyzed reaction takes place via unique radical mechanism, it will not only alternate with the conventional palladium-based process but also provide new applications in industry. |
|
 |
|
|
The results were published in Journal of the American Chemical Society and then highlighted asThe Top 20 Most Read Article in July 2010. |
|
| Suzuki–Miyaura coupling reaction The Suzuki–Miyaura coupling reaction is one of the C–C bond forming reactions, which can couple organoboron compounds with organic halides. It takes place under mild conditions and thus a variety of functional groups remain intact. Palladium is known to be an effective and versatile catalyst and often used for academic and industrial purposes. |
|
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center