Professor SHIMAKAWA, Y and his research group, Succeeded in Preparing “Single-crystal Thin Films of Infinite-layer Structure SrFeO2 with Square-planer Coordination of Fe2+ Ions”(Reported in 2 Sep 2008)
| Professor SHIMAKAWA, Yuichi and his research group (Laboratory of Advanced Inorganic Synthesis, Division of Synthetic Chemistry)
Succeeded in Preparing “Single-crystal Thin Films of Infinite-layer Structure SrFeO2 with Square-planer Coordination of Fe2+ Ions” |
 Prof Shimakawa Y(left), Mr Inoue S(center) and Mr Kawai M(right) |
||
| Mr. Satoru Inoue, Mr. Masanori Kawai, and Professor Yuichi Shimakawa in Advanced Inorganic Synthesis laboratory succeeded in preparing “single-crystal thin films of infinite-layer structure SrFeO2 with square-planer coordination of Fe2+ ions”. | |||
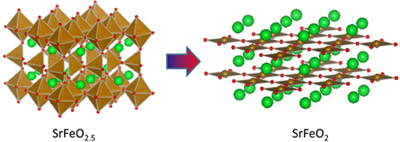 Figure 1. Crystal Structure of SrFeO2.5 and SrFeO2 |
There are a number of oxides with transition-metal ions such as Fe, Co, and Ni. Ionic states of the transition metals can vary in the oxides. For instance, Magnetite (Fe3O4) contains Fe2+ and Fe3+ions, while Hematite (Fe2O3), which used to be used as a red pigment, contains only Fe3+ ions. For strontium (Sr) and iron (Fe) containing perovskite-structure oxides, the oxygen content and Fe ionic state were considered to change between SrFeO3 and SrFeO2.5. SrFeO3 is a simple perovskite and it contains unusually high valence 4+ iron ion, which is stabilized by a strong oxidizing atmosphere. SrFeO2.5, on the other hand, is prepared at ambient pressure, and its brownmillerite structure consists of alternate layers of Fe3+ octahedra and tetrahedra. However, it was not possible to produce a perovskite with Fe2+ by using any reduction techniques. The brownmillerite SrFeO2.5 was thus historically assumed to represent the lower limit of oxygen nonstoichiometry in the perovskites. Last year a new compound, infinite-layer structure SrFeO2, was reported in Nature to be synthesized by using a low temperature reduction with CaH2. | ||
| Immediately after this report, Mr. Inoue and Mr. Kawai started the project for thin-film growth, and they succeeded in preparing “single-crystal thin films of infinite-layer structure SrFeO2”. A SrFeO2.5 precursor thin film was first deposited by a pulsed-laser-deposition method and the film was then reduced at low temperature with CaH2. The resultant sample was confirmed to be a single-crystal infinite layer SrFeO2 from X-ray diffraction and absorption experiments. | |||
| With the epitaxially grown thin-film samples, we can investigate mobile behaviors of oxygen ions. The results on high oxygen mobility will be useful for fuel-cell applications. The study on single-crystal thin-film samples will also reveal anisotropic crystal and electronic structures of the compound. New physical properties of the infinite-layer structure may appear by using epitaxial strain from the substrate lattice. The present success of preparing the single-crystal thin film SrFeO2 has great impacts on not only research fields of fundamental solid state physic and chemistry but also application fields of new material synthesis with new functions. |
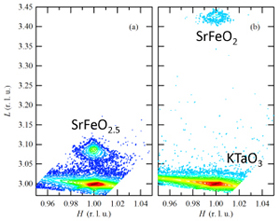 Figure 2. X-ray diffraction of SrFeO2.5 and SrFeO2 |
||

|
The research was done in collaboration with research groups of Profs. Kageyama and Yoshimura at Kyoto University and of Dr. Mizumaki at SPring-8/JASRI. The results were published in Applied Physics Letters and also presented in meetings of the Physical Society of Japan and the Japan Society of Applied Physics. | ||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center