Prof TOKITOH, N., Dr NAGAHORA, N., Assist Prof SASAMORI, T. and co-workers “Syntheses and Properties of Kinetically Stabilized 1,1´-Bis[(E)-diphosphenyl]ferrocenes” (Published in 15 Oct 2007)
| Professor TOKITOH, Norihiro, Dr NAGAHORA, Noriyoshi, Assistant Professor SASAMORI, Takahiro and their co-workers “Syntheses and Properties of Kinetically Stabilized 1,1´-Bis[(E)-diphosphenyl]ferrocenes” Published in Bulletin of the Chemical Society of Japan (BSCJ Award Article), October 15th, 2007 |
 |
|||
 |
Researchers at the Institute for Chemical Research and collaborators at Waseda Univ. have synthesized the first stable 1,1´-bis[(E)-diphosphenyl]ferrocenes bearing extremely bulky substituents and discovered their unique properties. | |||
|
The chemistry of heavier congeners of an azo-compound (-N=N-) has been studied extensively for more than 25 years, since the pioneering synthesis and isolation of the first stable diphosphene (-P=P-) bearing 2,4,6-tri-tert-butylphenyl groups reported by Yoshifuji and his co-workers. Nowadays, it is well-known that diphosphenes, which have relatively low-lying π* orbitals, readily undergo one-electron reduction to give their anion-radical species. |
||||
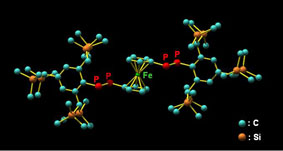
| We have reported the synthesis of novel doubly bonded systems between heavier group 15 elements (dipnictenes), that is, diphosphene (-P=P-), distibene (-Sb=Sb-), dibismuthene (-Bi=Bi-), phosphabismuthene (-P=Bi-), and stibabismuthene (-Sb=Bi-), by taking advantage of bulky protecting groups, such as 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl (Tbt) and 2,6-bis[bis(trimethylsilyl)methyl]-4-[tris(trimethylsilyl)methyl]phenyl (Bbt) groups (Figure 1). As an extension of the studies on novel d–π systems containing heavier group 15 elements, we have reported the syntheses, structural characterization, and properties of the first 1,1´-bis[(E)-diphosphenyl]ferrocenes (E,E)-1a and (E,E)-1b kinetically stabilized by the Tbt and Bbt groups, respectively. | 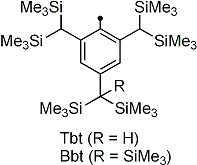 Figure 1. Tbt and Bbt groups as effective steric protecting groups developed by Tokitoh’s group. |
|||
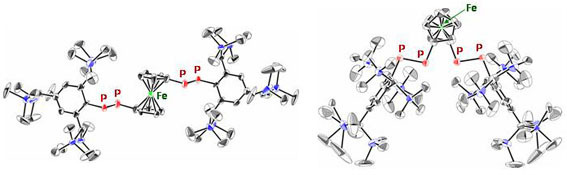
| Treatment of 1,1´-bis(dichlorophosphino)ferrocene with two equivalents of LiP(H)Ar (Ar = Tbt or Bbt) followed by the double-dehydrochlorination reaction using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) afforded (E,E)-1a and (E,E)-1b in 68% and 62% isolated yields, respectively (Scheme 1). The molecular structures of (E,E)-1a and (E,E)-1b were characterized by spectroscopy and X-ray crystallographic analyses (Figure 2). | ||||
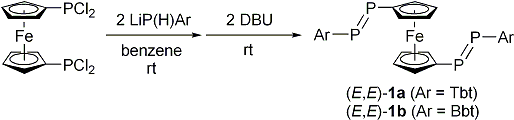 |
||||
| Scheme 1. Syntheses of (E,E)-1a and (E,E)-1b. | ||||
 |
||||
| Figure 2. ORTEP drawings of the major parts of (E,E)-1a (left) and (E,E)-1b (right) with thermal ellipsoid plots (50% probability). | ||||
| The UV–vis spectra of (E,E)-1a/(E,E)-1b in benzene showed three absorption maxima at 384/389 (ε = 7300/7400), 480/485 (sh, ε = 1800/1300), and 539/553 nm (ε = 2200/2200) as shown in Figure 3, which corresponded to the π(P=P)→π*(P=P), n(P=P)→π*(P=P), and d(Fe)→π*(P=P) electron transitions, respectively. In addition, the assignment of the observed absorption maxima for (E,E)-1a/(E,E)-1b was reasonably supported by theoretical calculations for the excited states of their symplified molecules. | 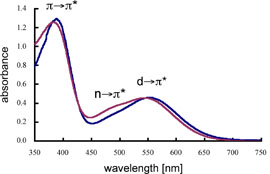 |
|||
| Figure 3. UV–vis spectra of (E,E)-1a (purple line) and (E,E)-1b (blue line) in benzene. | ||||
|
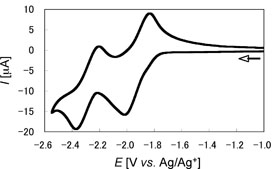
The electrochemical properties of (E,E)-1a and (E,E)-1b were revealed by cyclic and differential pulse voltammetries. It is remarkable that the two reversible one-electron redox waves due to the stepwise reductions of the P=P units of (E,E)-1aand (E,E)-1b were found in the range from about –1.8 to –2.4 V versus Ag/Ag+ (Figure 4). These results pointed out the electronic interaction between the diphosphene moieties through the central ferrocene units of (E,E)-1a and (E,E)-1b. |
 |
|||
| Figure 4. Cyclic voltammogram of (E,E)-1b in 0.1 M n-Bu4NBF4/THF solution at room temperature. | ||||
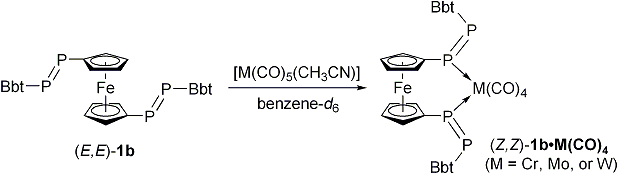
| Furthermore, we found that unique ligand exchange reactions of (E,E)-1b as a bidentate ligand with group 6 metals leading to the formation of novel 1,1´-bis[(Z)-diphosphenyl]ferrocene metal complexes [M(CO)4{(Z,Z)-(BbtP=PC5H4)2Fe}] (M = Cr, Mo, or W) (Scheme 2). | ||||
 |
||||
| Scheme 2. Ligand exchange reactions of (E,E)-1b with group 6 metals carbonyl complexes. | ||||
|
Further studies on elucidation of unique properties of novel π-conjugated systems having double bonds between heavier group 15 elements are in progress. |
||||
|
|
||||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center