Associate Professor MATUBAYASI, N; Professor NAKAHARA, M; and their coworkers, Established a Scheme of “Free-energy Analysis of the Molecular Binding into Lipid Membrane” (Published in 21 May 2008)
|
Associate Professor MATUBAYASI, Nobuyuki; Professor NAKAHARA, Masaru; and their coworkers Division of Environmental Chemistry, Laboratory of Solution and Interface Chemistry
Established a Scheme of “Free-energy Analysis of the Molecular Binding into Lipid Membrane” |
 Associate Prof Matubayasi N(left) and Prof Nakahara M(right) |
||
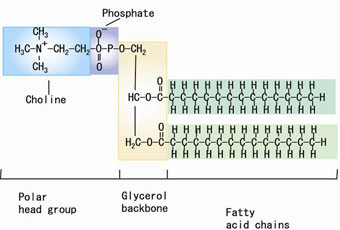 Figure 1. The molecular structure of DMPC (1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine) |
Phospholipid is a major component of biological membranes and is known to spontaneously form a bilayer in aqueous solution (Figure 1). The lipid membrane distinguishes one side of the solution from the other, and plays important roles in distribution and transport of a molecule. The molecular studies of lipid membranes have recently seen much progress, largely contributed by the advancement of the molecular dynamics simulation (MD) methodology (Figure 2). A key quantity to govern the membrane function is the binding free energy. However, the free-energy calculation in such complex systems as hydrated membranes is still difficult; since standard methods of free-energy calculation introduce several tens of “intermediate states” for computational convenience, their computation load is often prohibitive. This difficulty needs to be overcome to understand and control the molecular distribution and transport on the basis of the knowledge of intermolecular interactions. | ||
| The authors have established a new and fast scheme of free-energy calculation by combining a theory of solutions with MD. With the combination, the explicit treatment of “intermediate states” becomes unnecessary and the free energy can be computed several tens of times faster than the standard methods. The point is the formulation of a good theory of solutions to compute the free energy. The new theory developed by the authors is called the method of energy representation. The molecular theory of solutions has been studied for more than 70 years since 1930s. The tradition and common practice is the formulation in terms of the radial distribution function, which extracts the information of the distance distributions among atomic sites within molecule. It is long known, though, that a formulation in terms of the distance information is difficult to apply to frontline topics of physical chemistry such as supercritical fluid and nano-inhomogeneous solutions. The method of energy representation by the authors has introduced a new view and is formulated in terms of the information of the intermolecular interaction energy. With the new theory, supercritical fluid and nano-inhomogeneous solutions can be treated with good precision. By viewing lipid membrane and micelle as mixed solvent systems inhomogeneous in the nanometer-scale, it is further shown that a unified treatment of the subjects of interface chemistry is possible with the method of solution chemistry. |
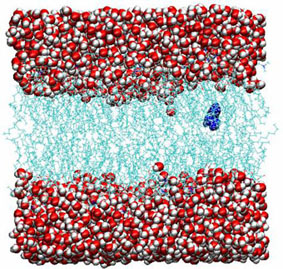 Figure 2. An MD snapshot of ethylbenzene trapped in hydrated DMPC bilayer. The DMPC molecules are located in the middle part and water is in the outer part. The ethylbenzene solute is denoted in blue. |
||
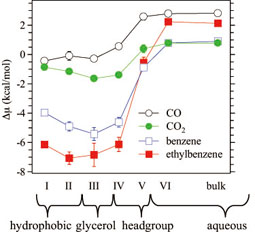 Figure 3. The free-energy change of inserting a hydrophobic solute into DMPC membrane. Six regions are introduced with an interval of 5 Å and are numbered I….VI from the membrane inside to outside. |
Figure 3 shows the free energy Δμ of binding of hydrophobic solute into DMPC bilayer. The Δμ calculation is now possible from all-atom potential functions using a relatively short MD. A hydrophobic solute is free-energetically stabilized within the membrane inside compared to bulk water. The stability is quite high even in the polar and hydrophilic headgroup region. This is due to the interaction with water present outside the membrane; the effect of excluded volume, which is the source of hydrophobicity, reduces drastically in the interfacial region, while the medium-range attraction by dispersion interaction persists. Corresponding experimental information, especially in the headgroup region, is now being obtained. It is also possible to calculate the membrane-water partition coefficient from Figure 3. This is a good step toward material design using soft, lipid membranes. | ||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center