Waning-and-Waxing Shape Changes in Ionic Nanoplates upon Cation Exchange
Published in “Nature Communications” (Online Publication, June 08, 2024)
(Advanced Inorganic Synthesis, Division of Synthetic Chemistry)
Associate Prof. Asaka, T.
(Nagoya Institute of Technology)
Flexible control of the composition and morphology of nanocrystals (NCs) over a wide range is an essential technology for the creation of functional nanomaterials. Cation exchange (CE) is a facile method by which to finely tune the compositions of ionic NCs, providing a valuable opportunity to obtain complex nanostructures that are difficult to form using conventional chemical synthesis procedures. However, due to their robust anion frameworks, CE cannot typically be used to modify the original morphology of the host NCs. In this study, we report an anisotropic morphological transformation of Cu1.8S NCs during CE. Upon partial CE of Cu1.8S nanoplates (NPLs) with Mn2+, the hexagonal NPLs are transformed into crescent-shaped Cu1.8S–MnS NPLs. Upon further CE, these crescent-shaped NPLs evolve back into completely hexagonal MnS NPLs. Comprehensive characterization of the intermediates reveals that this waxing-and-waning shape-evolution process is due to dissolution, redeposition, and intraparticle migration of Cu+ and S2−. Furthermore, in addition to Mn2+, this CE-induced transformation process occurs with Zn2+, Cd2+ and Fe3+. This finding presents a strategy by which to create heterostructured NCs with various morphologies and compositions under mild conditions.
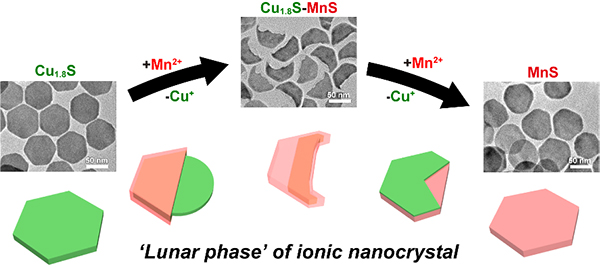
Figure : Lunar-phase-like waning-and-waxing morphological transformation of Cu1.8S nanoplates in cation exchange process.
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center