Determinants of Crystal Structure Transformation of Ionic Nanocrystals in Cation Exchange Reactions
Published in “Science” (Online Publication, July 16, 2021).
(Advanced Inorganic Synthesis, Division of Synthetic Chemistry)
Associate Prof. Asaka, T.
(Nagoya Institute of Technology)
Associate Prof. Tatetsu, Y.
(Meio University)
Ionic nanocrystals (NCs) have been widely used as photo-functional materials, whose properties are determined by the constituent elements and crystal structures. Cation exchange reaction has attracted much attention because it can easily modulate the composition of ionic NCs to prepare a variety of functional materials. However, it is believed that cation exchange hardly changes the crystal structure of parent ionic NCs.
We applied the cation exchange reaction to hexagonal-prism-shaped Cu1.8S NCs with 16 kinds of height and width using Co2+ cation. It was discovered that crystal system of resultant CoSx NCs depends on the height of parent Cu1.8S NCs, in which the original hexagonal-close-packed (hcp) crystal system of Cu1.8S NCs with thicker or thinner than about 10 nm yielded cubic-close-packed (ccp) Co9S8 or hcp CoS NCs, respectively (Figure). The ab initio calculation revealed the surface energy of side surface is larger than that of basal plane in hcp CoS, suggesting unfavored large side surface area of thick CoS NCs drove the phase transformation into more stable ccp Co9S8.
This discovery could lead to the phase control of ionic NCs under mild condition, which enables the synthesis of unexplored functional ionic nanomaterials.
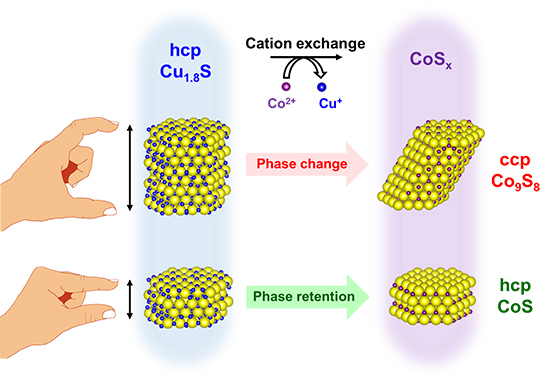
Figure. Thickness-dependent phase transformation of hcp Cu1.8S NCs during cation exchange reaction with Co2+.
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center