Isolating the Simplest Hydrated Acid
Published in “Science Advances” (Online Publication, April 22, 2017).
(Structural Organic Chemistry, Division of Synthetic Chemistry)
Assist Prof. Shimoaka, T.; Prof. Hasegawa, T.
(Solution and Interface Chemistry, Division of Environmental Chemistry)
Dissociation of an acid molecule in aqueous media is one of the most fundamental solvation processes but remains poorly understood in detail at the distinct molecular level. We conducted the high-pressure treatments of an open-cage fullerene C70 derivative with HF in the presence of H2O, and found that an unprecedented encapsulation of H2O-HF and H2O in addition to expected one were achieved. Restoration of the opening yielded the endohedral C70s, i.e., (H2O-HF)@C70, H2O@C70, and HF@C70 in macroscopic scales (Figure 1). The putting of an H2O-HF complex into the fullerene cage was a crucial step and it would proceed by the synergistic effects of “pushing from outside” and “pulling from inside”. The structure of the H2O-HF was unambiguously determined by the single crystal x-ray diffraction analysis. The NMR measurements revealed the formation of a hydrogen bond between the H2O and HF molecules without proton transfer even at 140 °C.
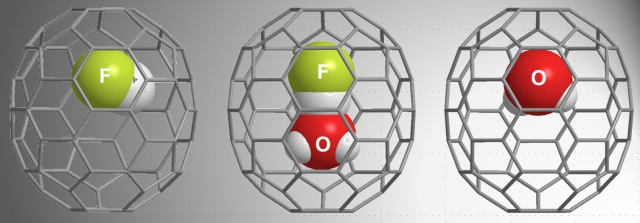
Figure 1. Molecular structure of endohedral fullerene C70 encapsulating HF, H2O-HF, and H2O.
Zhang, R.; Murata, M.; Wakamiya, A.; Shimoaka, T.; Hasegawa, T.; Murata, Y., Isolation of the Simplest Hydrated Acid, Science Advances, 3(4), e1602833 (2017).
DOI:10.1126/sciadv.1602833
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center