Germabenzenylpotassium: A Germanium Analogue of a Phenyl Anion
Published in “Angewandte Chemie International Edition” (March 8, 2017).
Selected as VIP (Very Important Paper) and Inside Cover.




Assist Prof. Mizuhata, Y., Ms. Fujimori, S., Assoc Prof. Sasamori, T., Prof. Tokitoh, N. (From Left)
Assist Prof. Mizuhata, Y.; Ms. Fujimori, S.; Assoc Prof. Sasamori, T.; Prof. Tokitoh, N.
(Organoelement Chemistry, Division of Synthetic Chemistry)
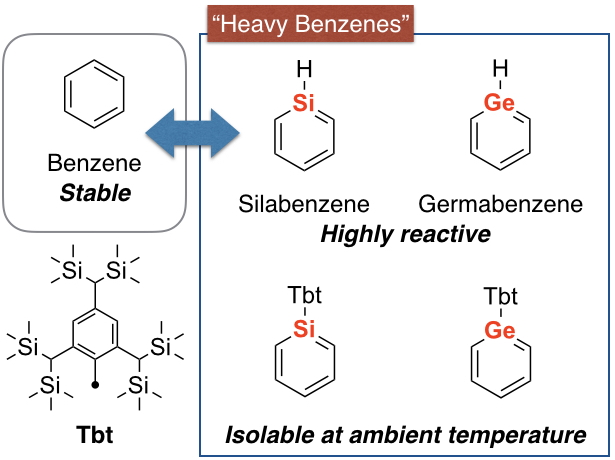
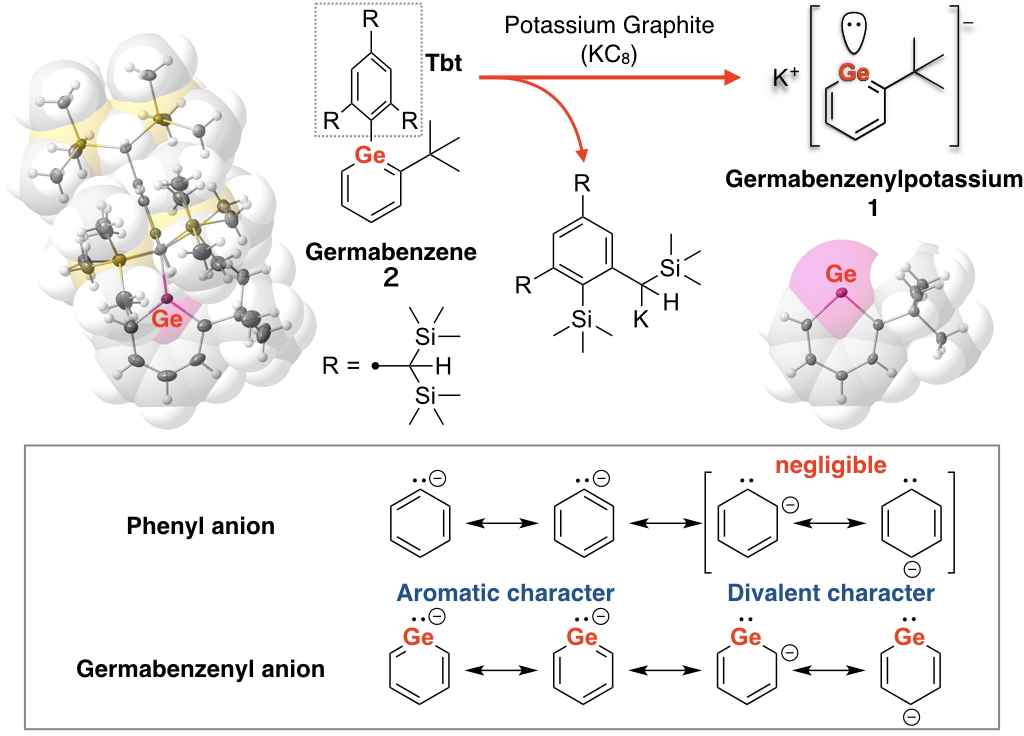
“Heavy benzenes” in which the carbon atom(s) of the benzene ring is replaced by high-period group 14 element(s), that is, “heavy element” (silicon, germanium, tin, and lead), have attracted much attention from the interest of its aromaticity experimentally and theoretically. However, these compounds are extremely high-reactive species, e.g., silabenzene (HSiC5H5) in which one of the skeletal carbon atom of the benzene ring is replaced by a silicon atom, is known to decompose by self-oligomerization reaction even at very low temperature of –200 ºC. We have already succeeded in the synthesis and isolation of a series of “heavy benzenes” as stable compounds even at room temperature by taking advantage of kinetic stabilization afforded by a very bulky substituent, Tbt group (see the figure), to prevent the self-oligomerization. Although these compounds are found to have “aromaticity” and unique electronic state, the existence of the bulky substituent necessary for stabilization makes it difficult to develop their further applications.
doi: 10.1002/anie.201700801
Published 8 March 2017
Inside Cover: Angew. Chem. Int. Ed., 56, 4364 (2017).
doi: 10.1002/anie.201702482
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center