Recently Published in Nature! “Nitrogen Reduction by the Fe Sites of Synthetic [Mo3S4Fe] Cubes”
In July 2022, our research paper was published in Nature. This study, entitled “Nitrogen reduction by the Fe sites of synthetic [Mo3S4Fe] cubes”, demonstrates the first catalytic reduction of N2 by synthetic metal-sulfur compounds and provides clues on how N2 is captured by the enzyme in nature and why the protein matrix around the N2-binding site is needed.
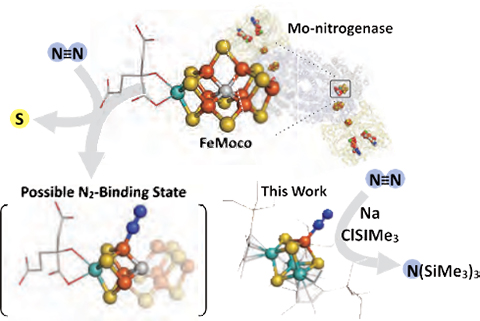
The reduction of N2 is the key elementary step to provide nitrogen atoms in amino acids and DNA and is hence indis- pensable for every form of life. Nitrogenase is the enzyme for this process, and at an atomic level, FeMoco (top in the figure) consisting of iron (Fe)-molybdenum (Mo)-sulfur (S)-carbon (C) is in charge of the difficult N2 reduction. Due to the structural complexity of FeMoco, the key elements of N2 reduction have remained unresolved. In this study, Ohki et al. predicted the structure of N2-bound FeMoco (left bottom of the figure) and the function of the protein, and synthesized cubic Mo-Fe-S compounds as simplified models (right bottom of the figure). This study not only represents the first step toward N2 reduction by artificial metal-sulfur compounds, but also a good example of how the utilities of metal-sulfur compounds can be expanded by learning from enzymes and applying appro- priate molecular design.
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center