Demonstration of Photoluminescence of Monovalent Indium Centres in Phosphate Glass − Novel Materials Field of Rare Element Indium −
This research topics is based on the studies published in “Scientific Reports“on September 1, 2015.
 |
 |
| Assist Prof. Masai, H. | Program-Specific Assoc Prof. Yamada, Y. |
Assist Prof. Masai, H.
(Inorganic Photonics Materials, Division of Materials Chemistry)
Assist Prof. Masai, H.,1 Program-Specific
Assoc Prof. Yamada, Y.,2 Mr. Okumura, S.,1 Prof. Yanagida, T.,3 Dr. Fujimoto, Y.,4 Prof. Kanemitsu, Y.5 and Dr. Ina, T.6 have demonstrated photoluminescence of metastable monovalent Indium (In+) species in oxide glasses.
1Inorganic Photonics Materials, Division of Materials Chemistry
2Nano-Interface Photonics (SEI Group CSR Foundation), presently, Assoc Prof in Chiba University
3NAIST
4Tohoku University
5Photonic Elements Science, International Research Center for Elements Science
6JASRI
Indium oxide is an important rare metal oxide that has been used in various applications, and transparent electrical conduction films such as ITO are essential components in functional devices. However, it is assumed that all In species in such materials are in the trivalent state, and the metastable monovalent In (In+) species has not been recognised as a component in oxide materials. On the other hand, In+ belongs to the ns2-type centre in phosphors. However, photoluminescence (PL) has not been studied for the In+ centre in powdered oxide phosphor because of the thermally metastable valence state.
In the present study, the group has examined the PL properties of zinc phosphate glasses containing In+ emissions centres (Figure 1). As shown in Figure 2, In-doped phosphate glass prepared by melt-quenching method exhibits the absorption bands (red line; ①-③). Black bands correspond to each PL excitation (PLE) band. Because these absorption bands were disappeared after the melting in air, it is expected that generated In+ was oxidized to stable In3+, which induced the change of both absorption and PLE bands. In addition, the X-ray absorption fine structure (XAFS) measurement in SPring-8 showed that the average atomic value of the In cation took lower valence state compared with In3+, and that the local coordination state changed with the composition of phosphate glass. On the other hand, the emission lifetime of the glass was on the microsecond scale, indicating a characteristic lifetime of ns2 type-center. Therefore, we have demonstrated the existence of In+ center in the phosphate glass.
It is notable that an absorption band ② exhibiting the highest excitation intensity is different from the strongest absorption band ③ (see Figure 2). Phosphorescence is usually observed from ① and ②(triplet excitation) states, which is obtained by excitation to ③(singlet excitation state) and the following energy relaxation to the triplet state. In other words, no emission of conventional crystals is usually observed by excitation to ① or ② states at room temperature. Such an energy state is characteristic of random oxide networks and has not been previously reported. Such capacity for a local structure is one of the merits of amorphous materials possessing the random oxide networks. Therefore, these findings will expand the science field of In-related materials.

Figure 1. Photograph of the In-doped glass irradiated with UV light (254 nm).
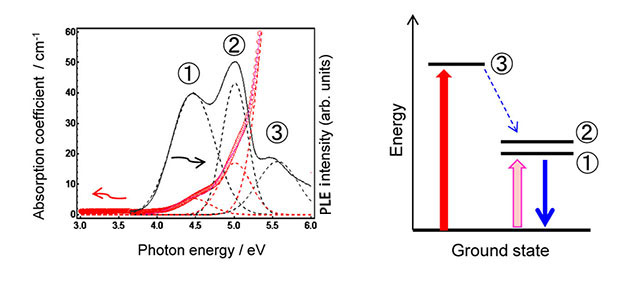
Figure 2. Optical absorption (red) and PLE (black) spectra of an In-doped zinc phosphate glass. Dashed lines show each absorption/ emission band after peak deconvolution. The figure indicates that clear emission of In+ was confirmed even by excitation of small absorption bands (① and ②). On the other hand, both the optical absorption edge and the PLE bands attributed to In+ species disappeared after melting in air because of oxidation to In3+.
Monovalent Indium (In+): Metastable valence state of indium whose existence in oxide materials has not been well-studied. In the phosphor field, In+ center belongs to ns2-type emission center because the electron configuration of the ground state is 5s2, which is the same as Sn2+ or Sb3+ center.
X-ray Absorption Fine Structure (XAFS) Measurement: One of the spectroscopic analysis using high-energy synchrotron radiation beam of a large synchrotron radiation facility, such as SPring-8. By using this method, information about atomic distance or valence state can be obtained.
This work was partially supported by a Grant-in-Aid for Young Scientists (A) (Grant #26709048), ICR Grants for Young Scientists, and the Collaborative Research Program of the Institute for Chemical Research, Kyoto University (Grant #2013-62 and #2014-31).
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center