Dr. Chen,W.-T., Assist Prof Saito,T., and Prof Shimakawa,Y., et. al. ”Revealing the Ligand-hole Localization Behaviors in Oxides with Unusual High-valence Fe” (Published in “Scientific Reports” 11 June 2012)
|
Revealing the Ligand-hole Localization Behaviors in Oxides with Unusual High-valence Fe
Published in Scientific Reports
Dr. Chen,W.-T., Assist Prof Saito,T., and Prof Shimakawa,Y., et. al. (International Research Center for Elements Science, Advanced Solid State Chemistry Laboratory ) |
Prof. Shimakawa,Y., Dr. Chen,W.-T., Assist.Prof. Saito,T. (from left) |
|
| Dr. Wei-tin Chen, Assistant Professor Takashi Saito, and Professor Yuichi Shimakawa in Institute for Chemical Research revealed the mechanism of the functional properties of oxides with unusual valence Fe. The paper was published in a Nature publishing group journal “Scientific Reports”. | ||
|
Iron ions in oxides usually show the +2 and +3 oxidation states typically seen in wüstite (Fe2+O), magnetite (Fe2+Fe3+2O4), and hematite (Fe3+2O3). A few oxides, such as SrFeO3 and CaFeO3, contain unusual high-oxidation-state iron ions like Fe4+, and the behaviors of such high-valence iron ions have been attracting much attention for a long time. |
||
| The research team recently discovered new such compounds; CaCu3Fe4O12 and LaCu3Fe4O12 with the A-site-ordered perovskite structure (see Figure 1). The compounds contain iron ions with unusual valence states and exhibit distinct electronic behaviors at low temperatures, e.g. charge disproportionation (4Fe4+→2Fe3++2Fe5+) in CaCu3Fe4O12 and intersite charge transfer (3Cu2++4Fe3.75+→3Cu3++4Fe3+) in LaCu3Fe4O12. In the present study, they made solid solutions of CaCu3Fe4O12 and LaCu3Fe4O12 and investigated their temperature-dependent transitions. Although the charge-disproportionation and intersite-charge-transfer behaviors look completely different from each other in simple ionic models, they can both be explained by the localization of ligand holes, which are produced by the strong hybridization of iron d and oxygen p orbitals in oxides. In the charge-disproportionated phase the ligand holes are localized at the Fe site and the transition is regarded as one to the rock-salt-type charge ordering of the ligand holes. In the intersite charge transfer, on the other hand, the ligand holes are localized at the Cu site and the transition can be regarded as a Mott transition of the ligand holes. TCT decreases with increasing concentration of the ligand-hole carriers (see Figure 2). | ||
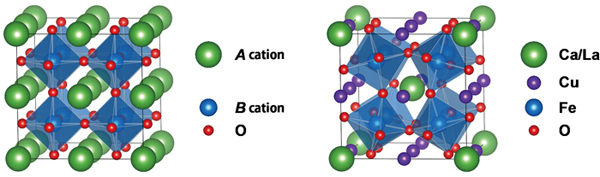
Figure 1. Crystal structures of the simple perovskite ABO3 (left) and the A-site-ordered double-perovskite AA’3B4O12(right). |
||
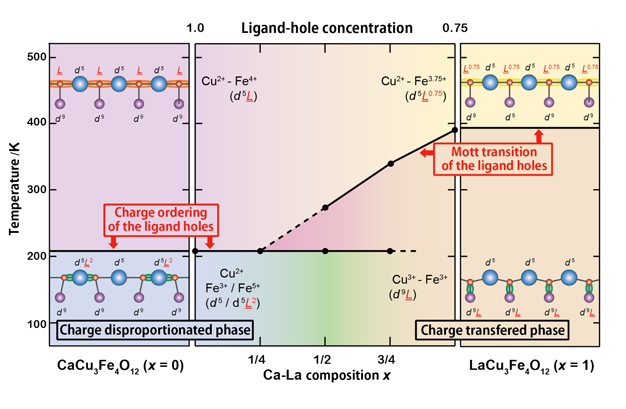
Figure 2. Compositional phase diagram for the Ca1−xLaxCu3Fe4O12 solid solution and the ligand-hole localization model of charge disproportionation and intersite charge-transfer transition behaviors. |
||
| In the A-site-ordered perovskite-structure oxides, transition metals at both A’ and B sites mediateA’-A’, A’-B, and B-B interactions that lead to intriguing physical properties. The ligand holes produced by the strong hybridization of transition-metal cation d orbitals and oxygen p orbitals also play important roles in giving rise to various electronic and structural properties. The present A-site-ordered perovskite-structure Ca1−xLaxCu3Fe4O12 solid solution is a novel example exhibiting interplay of the interactions mediated by the ligand holes. | ||
| The work was done in collaboration with Dr. Hayashi, N. (Micro/Nano Fabrication Hub) and Prof. Takano, M. (iCeMS). | ||
|
This work was supported in part by a Grant-in-Aid for Scientific Research 19GS0207, by the Global Centers of Excellence Program “International Center for Integrated Research and Advanced Education in Materials Science,” and by a grant for the Joint Project of Chemical Synthesis Core Research Institutions from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The work was also supported by the Japan Science and Technology Agency’s CREST program. |
||
| Chen, W.-T.; Saito, T.; Hayashi, N.; Takano, M.; Shimakawa,Y., Ligand-hole Localization in Oxides with Unusual Valence Fe , Scientific Reports, DOI:10.1038/srep00449 (2012). | ||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center