Prof Yuichi, Shimakawa and Rearch Group, ”Selective Reduction of Layers at Low Temperature in Artificial Superlattice Thin Films” (Published in Scientific Reports, 30 June 2011)
|
Selective Reduction of Layers at Low Temperature in Artificial Superlattice Thin Films
Published inScientific Reports(Online Publication, June 30, 2011). |
|
| Mr. Kazuya Matsumoto, Dr. Masanori Kawai, Ms. Aya Sakaiguchi, Dr. Noriya Ichikawa (Assis. Prof.), and Dr. Yuichi Shimakawa (Prof.) in Advanced Solid State Chemistry Laboratory, and Dr. Mitsutaka Haruta and Dr. Hiroki Kurata (Assoc. Prof.) in Electron Microscopy and Crystal Chemistry Laboratory found unusual behaviors of selective reduction of layers at low temperature in artificial superlattice oxide thin films. | |
|
Reduction and oxidation in transition-metal oxides is a key to develop technologies related to energy and the environment. Here we report the selective topochemical reduction observed when artificial superlattices with transition-metal oxides are treated at a temperature below 300 °C with CaH2. [CaFeO2]m/[SrTiO3]n infinite-layer/perovskite artificial superlattice thin films were obtained by low-temperature reduction of [CaFeO2.5]m/[SrTiO3]n brownmillerite/perovskite artificial superlattice thin films. By the reduction only the CaFeO2.5 layers in the artificial superlattices were reduced to the CaFeO2 infinite layers whereas the SrTiO3 layers were unchanged. The observed low-temperature reduction behaviors strongly suggest that the oxygen ion diffusion in the artificial superlattices is confined within the two-dimensional brownmillerite layers. The reduced artificial superlattice could be reoxidized, and thus, the selective reduction and oxidation of the constituent layers in the perovskite-structure framework occur reversibly. The results also give a potential application of the artificial superlattice as a two-dimensional oxygen reservoir. |
|
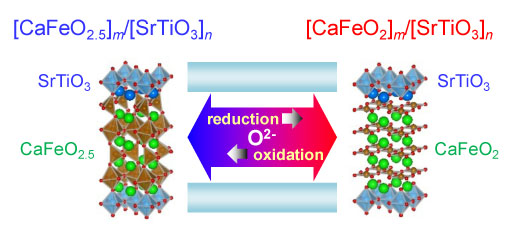
|
Selective reduction and oxidation of layers at low temperature in artificial superlattice thin films |
| This work was supported in part by a Grant-in-Aid for Scientific Research 19GS0207, by the Global Centers of Excellence Program “International Center for Integrated Research and Advanced Education in Materials Science”, and by a grant for the Joint Project of Chemical Synthesis Core Research Institutions from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The work was also supported by Japan Science and Technology Agency, CREST. | |
| The result was published in Scientific Reports (Online Publication, June 30, 2011). | |
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center