Atomic diffusion barriers and inter-element miscibility guide the development of unexplored crystal phases
Published in “Chemical Science” (Online Publication, September 10th, 2025)
(Advanced Inorganic Synthesis, Division of Synthetic Chemistry)
Dr. M. Kudo
(Kyushu University)
Associate Prof. Y. Tatetsu
(Meio University)
Crystal structure of a multicomponent alloy largely determines physical and chemical properties. A stabilization of unexplored crystal structures has been desired for developing novel- or high-functional materials. However, although many reports exist regarding the formation of metastable phases, there are few examples of unexplored crystal structures. Therefore, by elucidating the formation mechanism of the unprecedented Z3-type alloy structure we have recently succeeded in synthesizing, we believe that clues for exploring unexplored structures can be obtained.
In this study, we performed the reductive annealing for two-types of nanoparticles—FePd3 alloy with diffusion of In (FePd3:In nanoparticles) and PdInx alloy phase with diffusion of Fe (PdInx:Fe nanoparticles)—and discovered that the temperature required for synthesizing the Z3-type Fe(Pd, In)3 alloy nanoparticles was 200 K higher for the FePd3:In nanoparticles compared to the PdInx:Fe nanoparticles. To investigate this difference, we tracked the atomic diffusion process at the atomic level and found a difference in the adjacency of the immiscible element pair, Fe and In. Through first-principles calculations, we calculated the formation energies of intermediate structures formed in the two types of atomic diffusion processes, confirming that such adjacency between Fe and In greatly destabilizes the structure, thereby increasing the activation barrier for atomic diffusion.
These findings imply the necessity of controlling atomic diffusion processes in the exploration of unexplored alloy phases that contain immiscible element pairs, and they are expected to contribute to the promotion of future unexplored materials development.
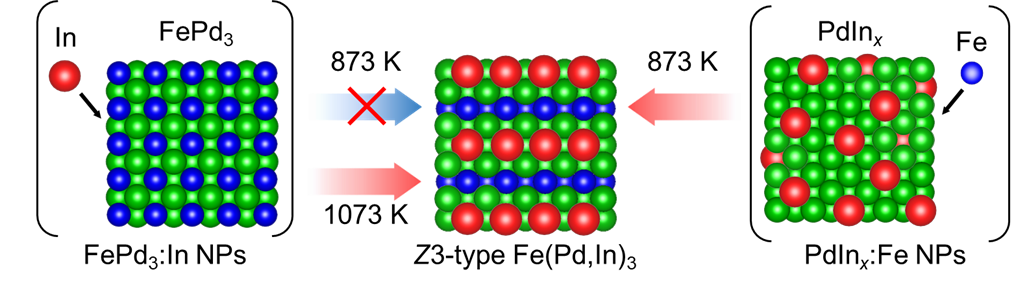
Figure : Differences in formation temperatures to form the Z3 phase derived from two-types of diffusion paths of the immiscible element pairs of Fe and In.
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center