|
Published in “Nature Chemistry“(Online Publication, March 7, 2016).
|

Assoc Prof.Wakamiya, A., Prof. Murata,T., Prof. Hasegawa, T., Dr. Ahren, T.,
Assist Prof. Murata, M., Mr. Zhang, R., Assist Prof. Shimoaka, T.
(clockwise from the one on the far left)
|
|
Prof. Murata, Y.; Mr. Zhang, R.; Assist Prof. Murata, M.; Dr. Aharen. T.;
Assoc Prof. Wakamiya, A.
(Structural Organic Chemistry, Division of Synthetic Chemistry)
Assist Prof. Shimoaka, T.; Prof. Hasegawa, T.
(Solution and Interface Chemistry, Division of Environmental Chemistry)
|
|
Water plays a critical role in a wide range of chemical and biological phenomena. The characteristic properties of bulk water such as high boiling and melting points are due to its hydrogen bond (H-bond) network. Thus, studies on intrinsic properties of a water monomer without any H-bond and a dimer with an H-bond are very important.
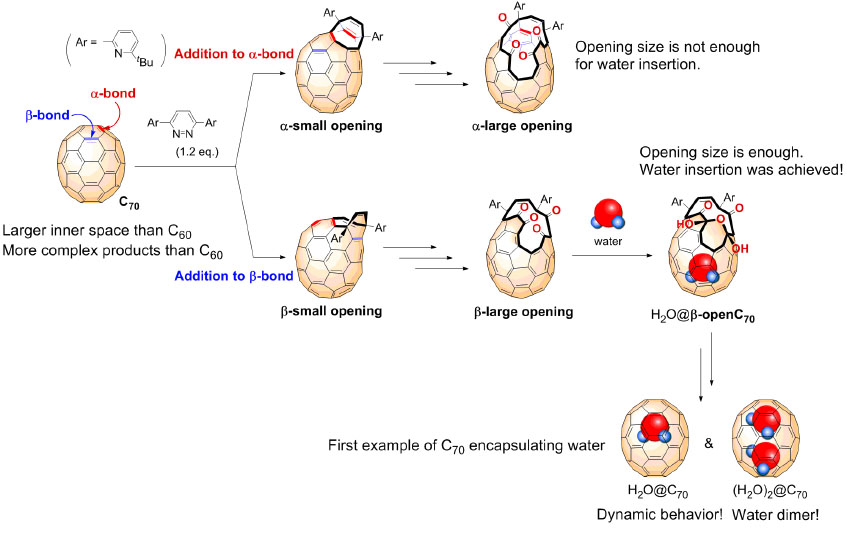
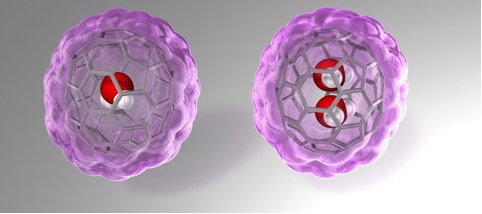
Fullerenes are spherical carbon clusters that have a hollow cavity. This sub-nano space is completely isolated from the outside environments by the outer carbon cage. Since fullerene C70 has a larger inner space than C60, C70 can accommodate two chemical species such as two He atoms and two H2 molecules. Here, Murata and co-workers report rational synthesis of H2O@C70 and unprecedented formation of (H2O)2@C70 with an isolated water dimer inside C70(Figure 1). Their strategically designed synthetic methods offer the successful encapsulation of one or two water molecules inside fullerene C70. These endohedral C70 represent the aforementioned condition of water and opened up the door to unveil intrinsic properties of a single water molecule as well as a genuine water dimer(Figure 2). The unambiguously determined off-centered position of water in H2O@C70 by X-ray diffraction provides insight into the formation (H2O)2@C70. Subsequently, the 1H NMR measurements for (H2O)2@C70 confirmed the formation of a single hydrogen bond rapidly interchanging between the dimeric water. Their theoretical calculations demonstrated a peculiar cis-linear conformation of the dimer achieved by confinement effects in C70. Endohedral fullerenes have been attracting much attention as functional materials for organic photovoltaics and bio-active materials. This report will open such application fields in near future.
|
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center