Synthesis of Aryl C-Glycosides via Iron-Catalyzed Cross Coupling of Halosugars: Stereoselective Anomeric Arylation of Glycosyl Radicals

Published in “Journal of the American Chemical Society”(Online Publication, August 1, 2017).
(J. Am. Chem. Soc. 2017, 139, 10693. DOI:10.1021/jacs.7b03867)
Assist Prof. Adak, L.; Dr. Kawamura, S.; Dr. Toma, G.; Dr. Takenaka, T.; Assist Prof. Isozaki, K.; Assoc Prof. Takaya, H.; Prof. Nakamura, M.
(Synthetic Organotransformation, International Research Center for Elements Science)
Mr. Li, H. C.; Prof. Shing, T. K. M.
(Department of Chemistry and Center of Novel Functional Molecules, The Chinese University of Hong Kong, China)
Prof. Orita, A.
(Department of Applied Chemistry, Okayama University of Science)
Aryl C-glycosides are of marked pharmaceutical interest due to their unique biological activities with high metabolic resistance toward enzymatic hydrolysis. Canagliflozin represents this class of aryl C-glycosides as an inhibitor for sodium-glucose cotransporter 2 (SGLT2), being commercialized as a drug of type 2 diabetes. In this study, a novel diastereoselective iron-catalyzed cross-coupling reaction of various glycosyl halides with organometallic reagents is developed to provide an efficient synthetic method for biologically and pharmaceutically important C-glycosides. A variety of aryl, heteroaryl, and vinyl metal reagents can be cross-coupled with C1-glycosyl halides in the presence of a catalytic amount of iron complex, FeCl2(TMS-SciOPP) to give various C-glycosides including Canagliflozin.
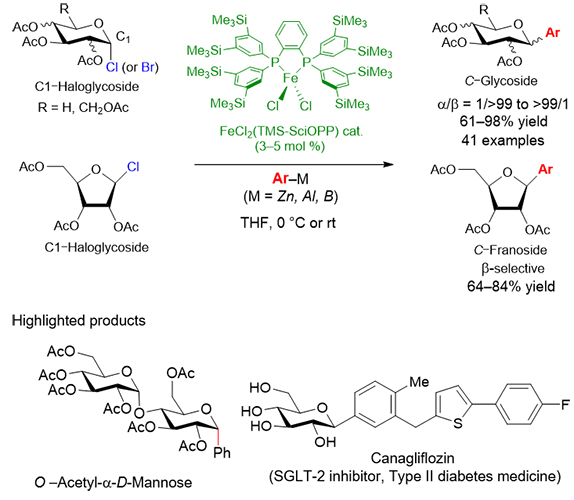
The key advantages of the present method over the previous C-glycosidation of glycosyl halides using Lewis acids, radical initiator/trapping agent, or nickel- and cobalt-catalyzed cross coupling are (1) structural diversity of the C-glycoside products, (2) high functional group compatibility, (3) wide applicability of organometallic reagents, and (4) the use of toxicologically and environmentally benign iron catalyst. In addition, the iron-catalyzed coupling reaction has been proven to proceed via the generation of glycosyl radical intermediates and their successive trapping with aryliron species. Theoretical study by using density functional theory (DFT) calculations shows that the observed diastereoselectivity can be rationalized by the relative stability of the conformers of the glycosyl radical intermediates. The structure/selectivity relationship will help further development of precisely controlled C-glycosidation reactions based on the iron catalyst. We hope that this simple, efficient, and practical synthesis of C-glycosides will provide a new entry to the development of a wide array of functional sugar derivatives of medicinal and clinical interest.
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center