Formation of pseudomorphic nanocages from Cu2O nanocrystals through anion exchange reactions
|
Published in “”Science” (Online Publication, March 18, 2016).
|
Dr. Wu, H.-L., Assist Prof. Sato, R.,
Mr. Yamaguchi, A., Assist Prof. Haruta, M.,
|
|
|
|
||
|
Dr. Wu, H.-L.; Assist Prof. Sato, R.; Mr. Kimura, M.; Prof. Teranishi, T.
Mr.Yamaguchi,A.; Assist Prof. Haruta, M.; Prof. Kurata, H. |
||
|
Ionic nanocrystals (NCs) have been widely used as photo-functional materials such as photocatalysts and photo-electric conversion materials, which are determined by the constituent elements, morphologies, and crystal structures. Because the stable crystal structures of the ionic crystals follow their phase diagrams, it has been difficult to chemically synthesize the high-temperature stable phases. |
||
|
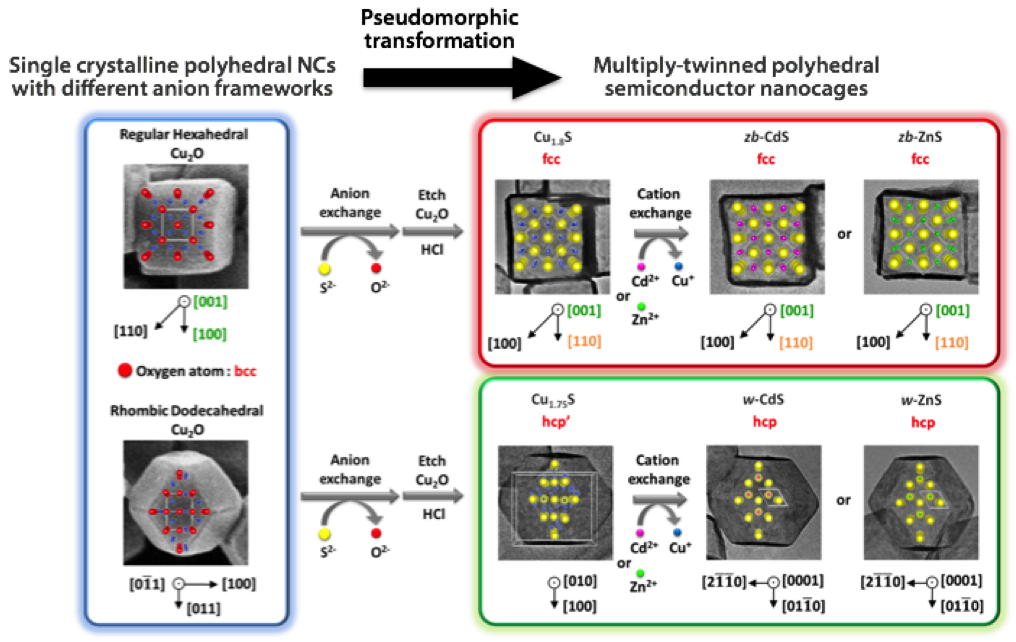
Figure. (Upper) The hexahedral Cu1.8S nanocages with a cubic phase are formed by the anion exchange (O2– → S2–) and the subsequent etching of hexahedral Cu2O NCs enclosed with {100} planes at ambient conditions. Further cation exchange (Cu+ → Cd2+, Zn2+) gives the hexahedral CdS or ZnS with a cubic phase. (Lower) The rhombic dodecahedral Cu1.75S nanocages with a triclinic phase are formed by the anion exchange (O2– → S2–) and the subsequent etching of rhombic dodecahedral Cu2O NCs enclosed with {110} planes at ambient conditions. Further cation exchange (Cu+ → Cd2+, Zn2+) gives the hexahedral CdS or ZnS with a hexagonal phase. |
||
|
|
||
 Institute for Chemical Research, Kyoto University
Institute for Chemical Research, Kyoto University International Joint Usage Research Center
International Joint Usage Research Center